Table of Contents
- 1. Introduction
- 2. Experimental Methodology for SO2 Adsorption
- 3. Comparative Analysis: SO2 Adsorption in Dry vs. Humid Gases
- 4. SO2/H2O Selectivity and Enhanced Adsorption
- 5. Results and Discussion: Implications for Industrial Desulfurization
- 6. Conclusion: Optimizing Activated Carbon for SO2 Removal
Introduction
Sulfur dioxide (SO2) emissions from industrial combustion processes contribute significantly to acid rain and environmental degradation. Activated carbon is a promising adsorbent for SO2 removal due to its hydrophobic properties and strong affinity for polar molecules. This article explores the efficiency of activated carbon in adsorbing SO2 from both dry and high-humidity exhaust gases, highlighting its potential for industrial applications.
Experimental Methodology for SO2 Adsorption
A dynamic breakthrough instrument simulated exhaust gas conditions to analyze SO2 adsorption. Activated carbon samples were placed in a test vessel with a gas space velocity of 530 h⁻¹. The temperature was maintained above 60°C using an oven and heating block. Gas flow was controlled by a mass flow controller, and dry or humidified nitrogen (N2) was used to dilute synthetic flue gas. Each experiment was repeated thrice to ensure consistency.
Comparative Analysis: SO2 Adsorption in Dry vs. Humid Gases
Experiments revealed that activated carbon exhibits a significantly higher affinity for SO2 compared to carbon dioxide (CO2) and N2. Notably, CO2 adsorption increased in wet gas conditions, peaking at 30°C. At higher temperatures, such as 60°C and 90°C, activated carbon demonstrated different adsorption capacities, with the SO2 adsorption rate higher in wet gas at 30°C and lower at 90°C. SO2 adsorption rates varied with temperature in humid conditions, indicating water’s role in enhancing SO2 capture under specific parameters.
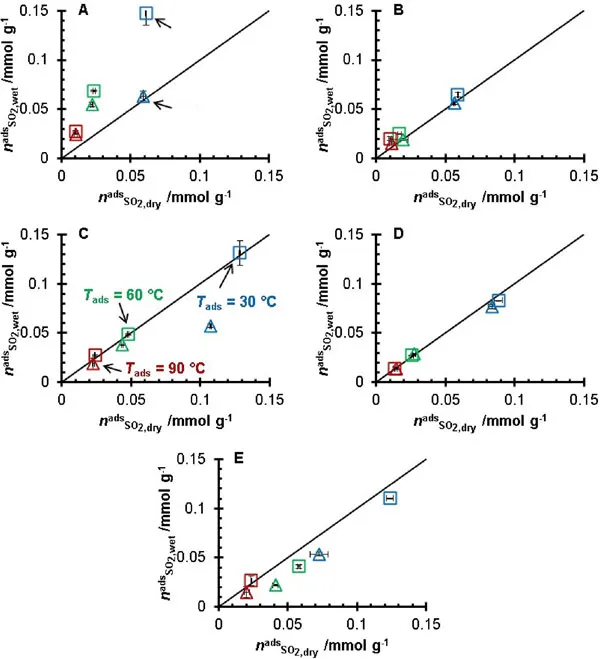
SO2/H2O Selectivity and Enhanced Adsorption
Activated carbon demonstrated high selectivity for SO2 in the presence of water vapor (H2O). Humid gas experiments showed increased SO2 adsorption, suggesting the formation of sulfuric acid or bisulfate salts on the carbon surface. Samples with higher oxygen content exhibited superior SO2 adsorption in wet conditions.
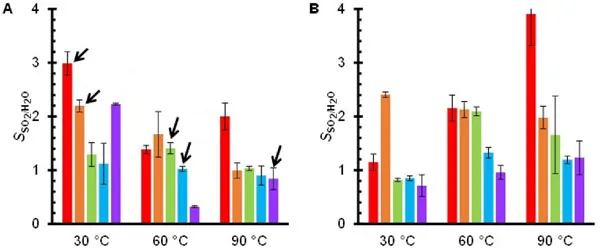
Results and Discussion: Implications for Industrial Desulfurization
The study confirms activated carbon’s effectiveness in adsorbing SO2 from both dry and wet exhaust gases, with a strong preference for SO2 over other gases. This high selectivity makes it ideal for industrial desulfurization, pellet and columnar activated carbon are widely used for industrial desulfurization. Activated carbon with higher oxygen content performed better in humid conditions, enhancing SO2 capture. Moreover, the material maintained its adsorption capacity after 10 cycles of wet SO2 exposure, indicating excellent reusability and stability for long-term industrial use.
Conclusion: Optimizing Activated Carbon for SO2 Removal
Activated carbon demonstrates excellent performance in SO2 removal from humid exhaust gases, with water enhancing its adsorption capacity, particularly in carbons with higher oxygen content. These findings suggest that optimized activated carbon is a valuable material for industrial desulfurization. Further research and development can enhance its efficiency and cost-effectiveness.
Article Keywords: activated carbon, SO2 adsorption, flue gas desulfurization, high humidity, sulfur dioxide removal, SO2/H2O selectivity, wet exhaust gas, dynamic breakthrough, industrial desulfurization, CO2 adsorption, activated carbon regeneration, air pollution control, exhaust gas treatment.